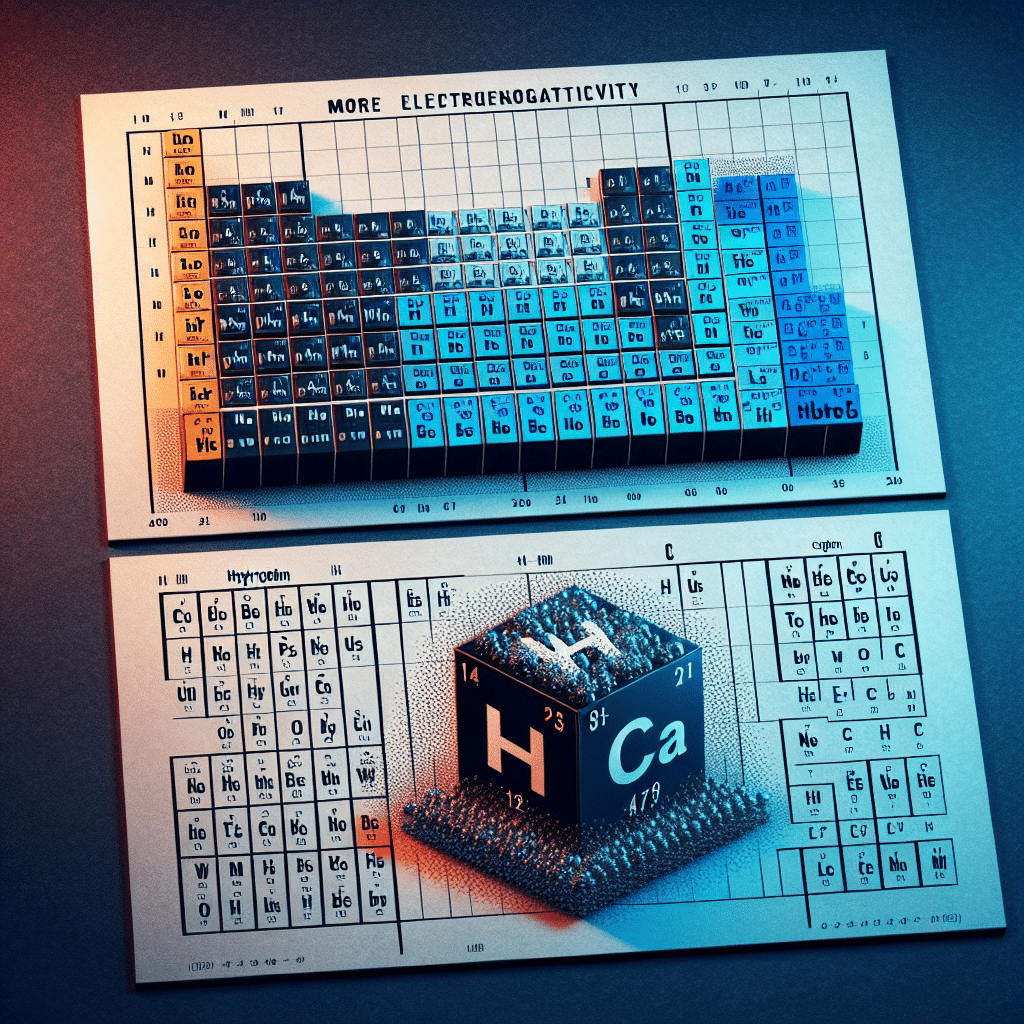
Electronegativity is a chemical property that describes an atom’s ability to attract and hold onto electrons. In comparing hydrogen (H) and carbon (C), we find that carbon is more electronegative than hydrogen. On the Pauling scale, carbon has an electronegativity value of about 2.55, while hydrogen’s value is approximately 2.20. This difference in electronegativity influences the behavior of molecules and compounds in which these elements are involved, including the types of bonds they can form. Understanding the electronegativity of these elements is crucial in fields ranging from organic chemistry to biochemistry, where they often participate in molecular structures.
Understanding Electronegativity
To fully grasp why carbon is more electronegative than hydrogen, it’s essential to dive deeper into the concept of electronegativity itself.
The Pauling Scale
The most widely used scale to measure electronegativity is the Pauling scale, developed by Linus Pauling in the early 20th century. This scale assigns values based on the ability of an atom in a molecule to attract electrons, with fluorine being the most electronegative element, assigned a value of 4.0.
Electronegativity Values
According to the Pauling scale, the values are as follows:
- Carbon (C): 2.55
- Hydrogen (H): 2.20
This variance indicates that carbon has a stronger attraction for electrons compared to hydrogen, which impacts their chemical interactions significantly.
The Implications of Electronegativity
Understanding the differences in electronegativity between hydrogen and carbon allows us to predict molecular behavior and bond characteristics.
Covalent Bonds
In compounds where both carbon and hydrogen are present, such as hydrocarbons, the difference in electronegativity leads to nonpolar covalent bonds. This means that when carbon and hydrogen share electrons, there is a relatively equal distribution of electron density. However, the small difference means that the bonds can exhibit slight polarization under certain conditions.
Reactivity and Stability
The electronegativity of carbon enables it to form stable bonds with various elements, which is critical in the formation of organic molecules. Because it is more electronegative, carbon can stabilize charged intermediates during chemical reactions, thus influencing the reaction pathways significantly.
Comparative Analysis: Hydrogen vs. Carbon
Let’s delve further into how this electronegativity difference manifests in various chemical phenomena and everyday applications.
Bonding Behavior
In organic chemistry, the electronegativity of carbon allows it to act as a central atom in complex molecules, forming chains and rings that serve as the backbone of organic compounds. Hydrogen, while less electronegative, plays a critical supportive role in stabilizing these structures. The following types of bonds are commonly evaluated:
- C-H Bonds: These covalent bonds are formed between carbon and hydrogen atoms, characterized by their relatively weak bond strength.
- Polar Covalent Bonds: When carbon bonds with more electronegative elements (like oxygen or nitrogen), it creates polar covalent bonds, significantly impacting the behavior of molecules in biochemical contexts.
Acidity and Basicity
The differences in electronegativity also have implications for acidity and basicity in chemistry. For example, in organic acids, the presence of electronegative atoms can stabilize negative charges after deprotonation, a factor heavily influenced by the surrounding electronegative atoms, including carbon.
Biological Significance
In biological systems, carbon’s electronegativity plays a role in enzyme function and metabolic pathways. The configuration of carbon-based molecules is crucial for interactions with enzymes, affecting everything from nutrient absorption to energy production.
FAQs
1. What are some examples of compounds involving carbon and hydrogen?
Common examples include gasoline (a mixture of hydrocarbons) and simple sugars like glucose, all containing both carbon and hydrogen atoms.
2. How does electronegativity affect the properties of water?
Water’s polar covalent bonds result from the electronegativity difference between hydrogen and oxygen, making water a universal solvent with unique properties.
3. Can the electronegativity of hydrogen change in different compounds?
While the electronegativity value of hydrogen remains constant, its apparent behavior may change depending on the electronegativity of surrounding atoms.
4. Why is carbon known as the backbone of life?
Carbon’s ability to form stable bonds with many elements allows it to create diverse organic molecules, essential for the structure and function of living organisms.
5. What role does electronegativity play in environmental chemistry?
Electronegativity differences contribute to the reactivity patterns of pollutants, influencing their behavior in biological systems and ecosystems.
Conclusion
In summary, understanding the concept of electronegativity and the relative values of hydrogen and carbon is crucial for predicting and explaining chemical behavior. While carbon is more electronegative than hydrogen, leading to significant implications in bonding and molecular chemistry, both elements play key roles in the composition of biological and inorganic compounds alike.