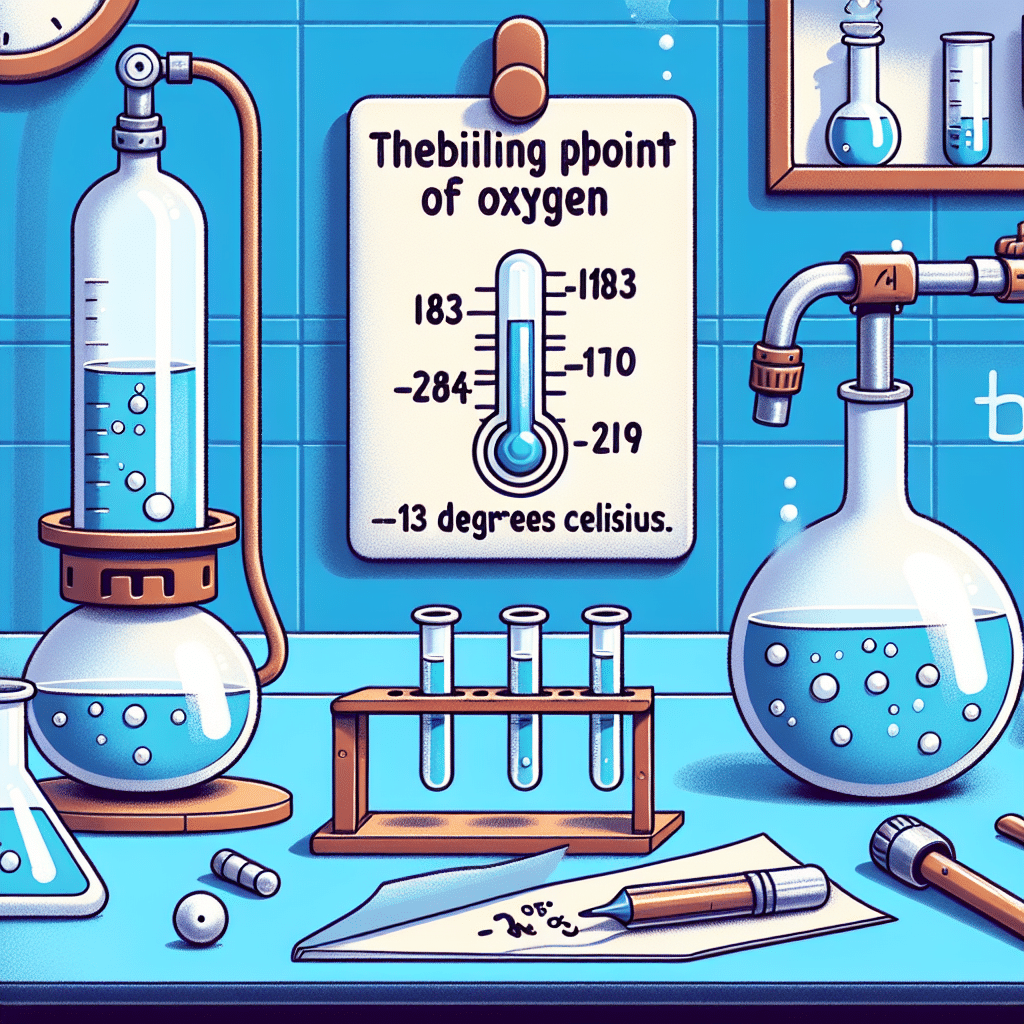What is the boiling point of oxygen?
The boiling point of oxygen at standard atmospheric pressure (1 atmosphere) is approximately -183 degrees Celsius (-297 degrees Fahrenheit). This is the temperature at which oxygen transitions from a liquid state to a gaseous state. Under normal atmospheric conditions, oxygen exists as a colorless, odorless gas, but when cooled to its boiling point, it transforms into a pale blue liquid. Understanding oxygen’s boiling point is crucial in various scientific and industrial applications, including cryogenics, where liquefied gases are utilized for their unique properties at low temperatures.
Introduction to Oxygen’s Boiling Point
Oxygen, a vital element for life, plays an essential role in combustion, respiration, and various chemical processes. Knowing its boiling point is not merely an academic curiosity; it has practical implications across several fields, from medicine to aerospace. The transition of oxygen from gas to liquid state at -183°C enables its use in liquid form for efficient storage and transport, especially in medical and industrial settings.
The Science Behind Boiling Points
Understanding Phase Changes
A substance’s boiling point is determined by a balance of vapor pressure and atmospheric pressure. When a liquid’s vapor pressure equals the surrounding atmospheric pressure, boiling occurs. For oxygen, this transition happens at a boiling point of -183°C at 1 atm. However, this boiling point can change with variations in external pressure, such as in high-altitude environments where atmospheric pressure is reduced.
Factors Influencing the Boiling Point
Several factors can influence the boiling point of a substance including:
- Pressure: Boiling points increase with increased ambient pressure and decrease with lowered pressure.
- Intermolecular Forces: The strength of intermolecular forces also plays a significant role. Oxygen molecules are held together by Van der Waals forces, which require a specific amount of energy to overcome, leading to its boiling point.
- Impurities: The presence of impurities in a liquid can elevate its boiling point through boiling point elevation.
Properties of Liquid Oxygen
Liquid oxygen is a cryogenic liquid with notable properties:
Physical Characteristics
Liquid oxygen is light blue, highly fluid, and has a density of around 1.14 g/cm³, which is significantly denser than gaseous oxygen. One of the noteworthy aspects of liquid oxygen is its high oxygen concentration, which can facilitate rapid combustion when combined with fuels.
Safety Considerations
Due to its strong oxidizing properties, liquid oxygen can pose risks of fire and explosion. Materials that are normally non-flammable can combust in the presence of liquid oxygen. Safety protocols are necessary when handling liquid oxygen in industrial applications to minimize the risk of fire hazards.
Applications of Liquid Oxygen
Understanding the boiling point and properties of liquid oxygen leads to numerous applications:
Aerospace and Propulsion
In the aerospace industry, liquid oxygen is often used as an oxidizer in rocket propellants. Its high energy content and capability to generate thrust make it invaluable in launching spacecraft into orbit.
Cryogenics
In cryogenics, liquid oxygen serves as a coolant in various applications, such as preserving biological samples and conducting scientific experiments at ultra-low temperatures.
Medical Uses
Liquid oxygen is crucial for medical oxygen supply systems, particularly in hospitals and emergency services, facilitating patient care in respiratory therapies.
Frequently Asked Questions (FAQ)
1. What happens to oxygen when it is cooled below its boiling point?
When oxygen is cooled below its boiling point of -183°C, it condenses from a gas into a pale blue liquid. This state remains stable until the temperature rises above the boiling point.
2. How is liquid oxygen produced?
Liquid oxygen is produced through a cryogenic distillation process, where air is cooled to extreme temperatures and various gases, including nitrogen and argon, are separated from oxygen based on their boiling points.
3. Can oxygen boil at higher temperatures?
Yes, the boiling point of oxygen can change with variations in atmospheric pressure. At higher pressures, the boiling point will increase above -183°C.
4. What safety measures should be taken when handling liquid oxygen?
Safety measures include using appropriate personal protective equipment (PPE), ensuring good ventilation, keeping flammable materials away, and storing liquid oxygen in proper containers designed to withstand the low temperatures.
5. Is liquid oxygen environmentally friendly?
Yes, liquid oxygen itself is a clean oxidizer and does not produce harmful emissions when combusted. However, the extraction and processing can have environmental impacts depending on the methods used.
Conclusion
The boiling point of oxygen, at approximately -183°C at standard atmospheric pressure, is a significant scientific detail that underscores its properties, uses, and safety considerations. By comprehending both the boiling point and the behavior of oxygen in its various states, professionals and enthusiasts alike can appreciate the nuanced roles this essential element plays in our world. Whether in aerospace exploration, medical applications, or industrial utilization, the relevance of liquid oxygen is expansive and ever-growing.